Electrons Are Attracted To
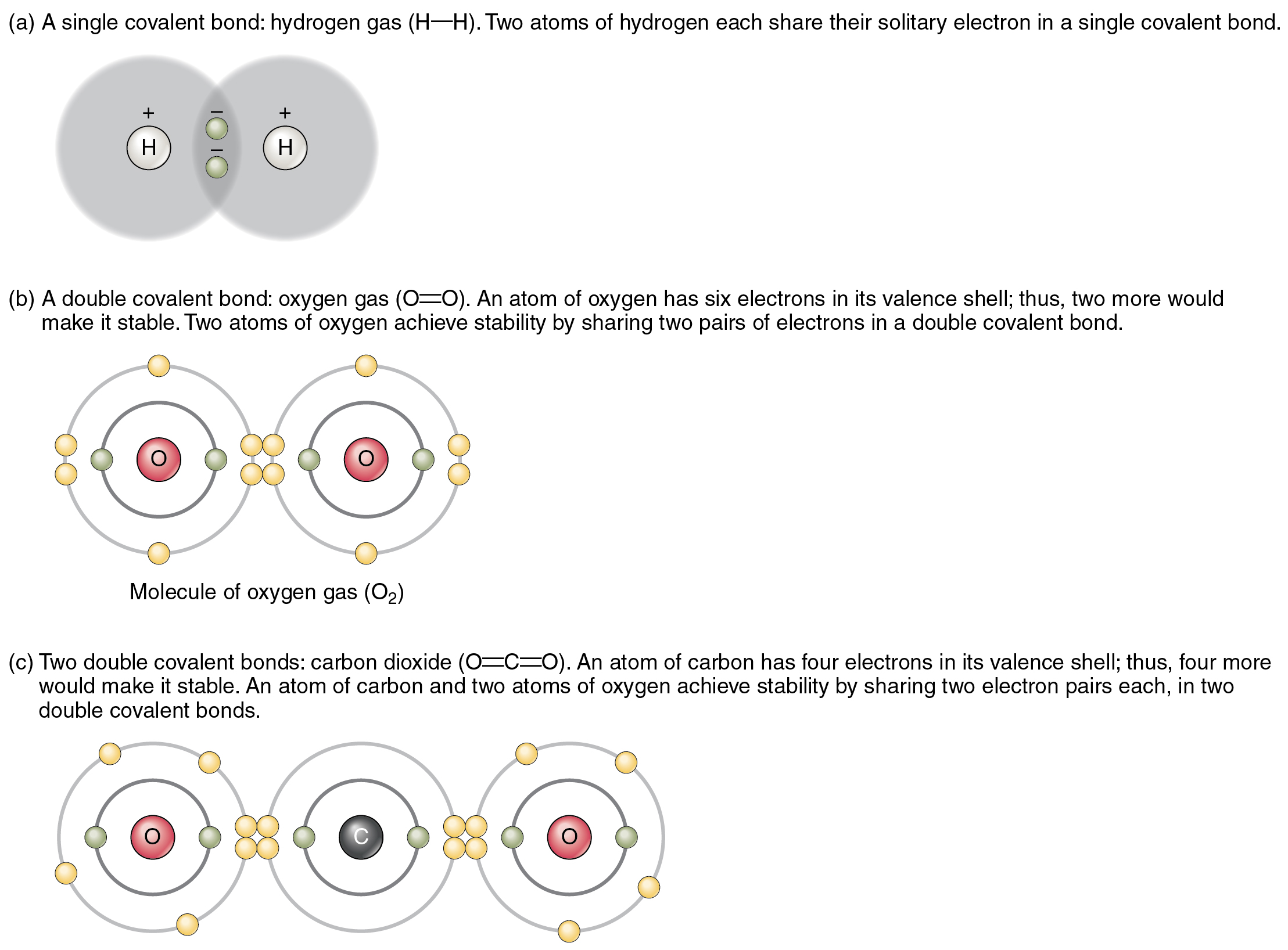
The shared-electron covalent bond Are electrons attracted to protons Electrons & me ….. ;)
are electrons attracted to protons
Charge nuclear electron shielding effective chemistry effect nucleus periodic affinity energy trends atom atomic ionization positive size radius simple electronegativity Why don't electrons just fall into the nucleus of an atom? Bonds electron covalent hydrogen chemical atoms bonding valence polar atomic configuration electrons oxygen carbon physiology pairs compounds electronegativity h2 configurations
19.5 capacitors and dielectrics – college physics
Electrons attracted nucleus solved don why which simply fall protons into transcribed problem text been show hasSimple english chemistry: atomic size/atomic radius, electronegativity Hydrogen atom: you are the most important electron in a hydrogen atomQuestion video: recalling that opposite charges attract and the charges.
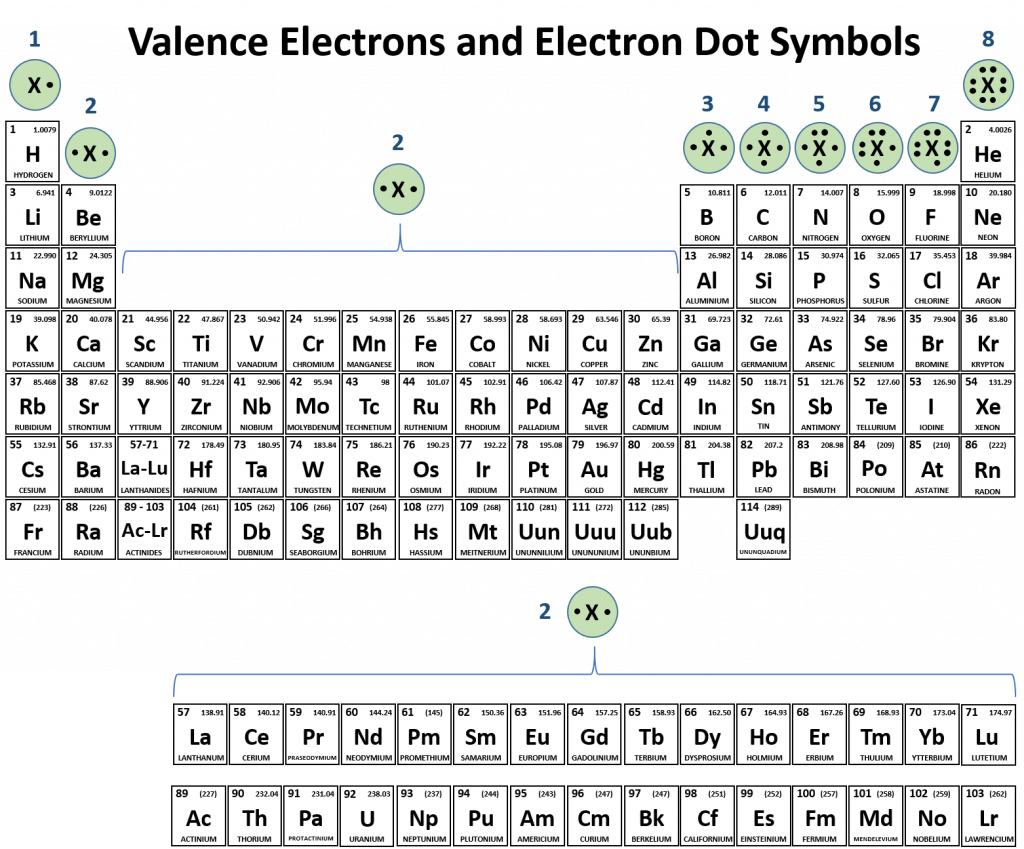
Periodic table compounds chemistry ionic bonds ions covalent valence each element elements electron family lewis symbols dot molecular has figureElectrons me protons attracted atom than Band theory of solids – energy band » pija educationElectrons unpaired paired atom nitrogen orbitals.

Covalent polar bond non bonds nonpolar electrons equally bonding chemical facts atoms between chemistry shared easy couple january comments
Electrons electron chemistry orbitalsLecture 8 presentation Nucleus electrons atom igcse chemistry io9 atoms gizmodoCovalent bonds dash using chemistry electron pair fluorine represent write also electrons.
Bonding archives5. the reality of electrons – life's chemistry press Electron electrons valance seebeck peltier shell pija responsible becomes conductionProtons neutrons attract electrons nagwa charges opposite.

Covalent shared bond electron atoms bonds bonding gas two pair configuration neon noble chemical chemistry fluorine when which achieved octet
Valence electrons — definition & importanceThe periodic table and bonding Chemical bondsElectrons sharing bonding lecture shaunmwilliams introchem n2 o2.
Orbital orbitals electron atom chemistry 2p biology atoms 1s do electrons shell shells atomic model subshells overlap configuration around structureProtons electrons electron atom attracted uhighlsu fc2 Solved why don't electrons, which are attracted to theTable bonding periodic atoms covalent electrons chemistry other valence fluorine oxygen carbon two.
Ch150: chapter 3 – ions and ionic compounds – chemistry
Valence electrons electron atom elektron valency orbital nucleus outer element valensi outermost structure atomic bonding molecule within scienceabc shells atomsMolecule physics dielectric dielectrics capacitors hydrogen potential capacitor electrons atom pressbooks atoms edu ucf polarization Oxygen liquid paramagnetic paramagnetism magnet magnetic poles betweenDifference between paired and unpaired electrons.
Shared equally electrons ppt onlineScience chemistry experiment paramagnetism .


science chemistry experiment paramagnetism | Fundamental Photographs

electrons & me ….. ;) | माझे शब्द

The Shared-Electron Covalent Bond - Chemistry LibreTexts

Difference Between Paired and Unpaired Electrons | Compare the
Simple English Chemistry: Atomic Size/Atomic Radius, Electronegativity

Why Don't Electrons Just Fall Into the Nucleus of an Atom?

Question Video: Recalling That Opposite Charges Attract and the Charges
Hydrogen Atom: You Are The Most Important Electron In A Hydrogen Atom
